Ajimoye
SubscriberForum Replies Created
-
What other on-site data collection tasks in aquaculture could benefit from similar mobile AI tools?
-
Appreciated
-
Muhammad Ahmad
MemberOctober 31, 2025 at 12:08 am in reply to: Moisture Loss at Silo for Maize Storage13-14%
-
There is a lot to be learned…. Thanks for the responses friends…
-
Informative write-up
-
Maintain maize grain moisture between 12.5 ± 0.5% during silo storage, allowing ≤ 1% moisture loss over the storage period for best quality and minimum economic loss.
Sakthivel V P
-
This reply was modified 1 month, 2 weeks ago by
 India.
India.
-
This reply was modified 1 month, 2 weeks ago by
-
Aeration Fan Operation – Best Practices for Mold & Moisture Control
Aeration fans should be used strategically to maintain cool, uniform grain temperatures and prevent moisture build-up. Operate fans during the coolest and driest times of day, typically between 10 PM and 7 AM, when ambient air temperature is low and relative humidity (RH) is below 70 %. Cool night air helps remove residual field heat and equalize temperatures throughout the silo.
Avoid running fans during humid, foggy, or rainy weather, as warm or moist air can add moisture to grain and create conditions for mold growth. Begin aeration when grain temperature exceeds ambient by 4–6 °C, and continue until the difference (ΔT) narrows to 2–3 °C. This prevents hot spots and reduces insect activity.
During the post-harvest period, continuous aeration for 2–3 days helps remove field heat. Thereafter, use intermittent aeration runs (4–6 hours each) to maintain uniform temperature. In warmer months, short night-time aeration cycles prevent heating at the top layers.
For optimal efficiency, equip silos with automatic aeration controllers linked to temperature and humidity sensors. Such systems activate fans only when conditions are favorable, conserving power while safeguarding grain quality.
Sakthivel V P
-
Preventive Approaches to Reduce Mold & Mycotoxin Risk in Grain Storage Silos
Preventive Approaches to Reduce Mold & Mycotoxin Risk in Grain Storage Silos 1. Grain Quality at Intake
• Preventive Actions: Accept only properly dried, clean, and uninfested grains. Remove broken, immature, or moldy kernels before storage.
• Key Notes / Parameters: Moisture ≤ 13–13.5% for maize; impurities ≤ 2%.
2. Pre-Drying & Moisture Control
• Preventive Actions: Dry grains quickly after harvest. Use mechanical dryers or aeration to reduce surface moisture. Avoid over-drying (grain cracking).
• Key Notes / Parameters: Target equilibrium moisture: 12–13%.
3. Temperature Management
• Preventive Actions: Maintain uniform grain temperature. Aerate to prevent hot spots. Use temperature cables and ΔT monitoring.
• Key Notes / Parameters: ΔT (grain vs ambient) ≤ 2–3°C.
4. Aeration & Ventilation
• Preventive Actions: Operate aeration fans during cool, dry periods. Ensure even airflow distribution. Periodically reverse air direction if possible.
• Key Notes / Parameters: Keep relative humidity of inlet air < 70%.
5. Hygiene & Sanitation
• Preventive Actions: Clean silos, conveyors, pits, and augers before filling. Remove old residues and dust. Sanitize with approved fumigants/insecticides.
• Key Notes / Parameters: Avoid cross-contamination from old stocks.
6. Insect & Pest Control
• Preventive Actions: Inspect regularly and treat as per schedule. Maintain sealed silo to prevent pest entry.
• Key Notes / Parameters: Pests increase moisture and fungal activity.
7. CO₂ & Moisture Monitoring
• Preventive Actions: Use CO₂ sensors to detect biological activity early. Check for condensation near walls and roof.
• Key Notes / Parameters: Sudden CO₂ rise → fungal activity alert.
8. Regular Sampling & Testing
• Preventive Actions: Perform monthly grain sampling for mold count and mycotoxin screening (Aflatoxin, DON, Fumonisin, etc.).
• Key Notes / Parameters: Keep logbook and trend data for traceability.
9. Rotation & FIFO Practice
• Preventive Actions: Implement First In–First Out. Avoid prolonged storage of one batch.
• Key Notes / Parameters: Prevents aging and fungal growth.
10. Use of Mold Inhibitors
• Preventive Actions: Apply organic acids (propionic, acetic) or commercial mold inhibitors if long-term storage is expected.
• Key Notes / Parameters: Ensure even distribution during loading.
11. Weather & Humidity Monitoring
• Preventive Actions: Record daily ambient humidity and temperature. Avoid aeration during humid or rainy weather.
• Key Notes / Parameters: Use automated weather-linked aeration control.
12. Roof & Seal Integrity
• Preventive Actions: Inspect for leaks, condensation, and poor insulation. Maintain waterproof seals and vents.
• Key Notes / Parameters: Prevents moisture ingress and caking
Sakthivel V p
-
Silo Storage Efficiency of Maize refers to how effectively a silo system maintains grain quality, minimizes losses, and optimizes storage costs over time. Efficient storage ensures the maize remains safe for feed production with minimal deterioration, insect infestation, or moisture migration.
Best Practices for Maize Silo Storage
Ø Dry grain uniformly to below 13% before filling the silo.
Ø Regularly monitor grain temperature and moisture (automated probes preferred).
Ø Use aeration fans during cool night hours for temperature equalization.
Ø Maintain a temperature logbook and record ΔT trends.
Ø Inspect roof vents and aeration ducts for blockage or corrosion.
Ø Rotate stored grain lots (FIFO) to avoid long-term stagnation.
Ø Fumigate periodically and ensure insect control protocols.
Sakthivel V P
-
Grain Temperature Difference (ΔT) in Silo Storage
Definition:
Grain Temperature Difference, denoted as ΔT, refers to the difference between the temperature of the stored grain mass and the surrounding ambient air temperature.
This parameter is a key indicator of the thermal condition and biological activity inside the silo.
Practical Monitoring Guidelines
Ø Install temperature cables at different heights and zones inside the silo.
Ø Record grain temperature and ambient temperature daily or weekly.
Ø Investigate if ΔT exceeds 5°C, and aerate immediately.
Ø Keep ΔT < 3°C for long-term safe storage.
Ø Maintain logbook or digital monitoring system for trend analysis.
Sakthivel V P
-
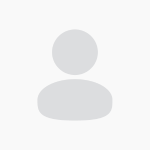
This table outlines standardized conversion factors between active substances and their corresponding vitamin forms, facilitating accurate nutrient quantification in feed and premix formulations. These values are essential for ensuring compliance, efficacy, and consistency across diverse ingredient sources.
Key Conversion Highlights
-
Betaine
-
1 mg active = • 2.13 mg liquid betaine anhydrous (47%) • 1.41 mg betaine hydrochloride (70%) • 2.38 mg choline chloride (60%)
-
-
L-Carnitine
-
1 mg active = 1.49 mg L-carnitine L-tartrate
-
-
Choline Chloride
-
1 mg active = 1.34 mg choline chloride (based on choline hydroxyl analogue)
-
-
Niacin
-
1 mg active = 1 mg nicotinic acid or 1 mg nicotinamide
-
-
D-Pantothenic Acid
-
1 mg active = 1.087 mg calcium D-pantothenate
-
-
Vitamin A
-
1 IU = • 0.3 μg retinol (retinyl acetate) • 0.344 μg retinyl palmitate
-
-
Vitamin B₁ (Thiamine)
-
1 mg active = • 1.27 mg thiamine mononitrate • 1.13 mg thiamine hydrochloride
-
-
Vitamin B₂ (Riboflavin)
-
1 mg active = 1.19 mg riboflavin 5’-phosphate sodium
-
-
Vitamin B₆ (Pyridoxine)
-
1 mg active = 1.21 mg pyridoxine hydrochloride
-
-
Vitamin D₃ (Cholecalciferol)
-
1 IU = 0.025 μg 25-OH-vitamin D₃
-
-
Vitamin E
-
1 IU = • 0.67 mg all-rac-alpha-tocopheryl acetate (25-OH-calciferol) • 1.1 mg RRR-alpha-tocopheryl acetate • 1.49 mg RRR-alpha-tocopherol • 2.22 mg all-rac-alpha-tocopherol
-
-
Vitamin K₂ (Menadione)
-
1 mg active = • 1.92 mg menadione sodium bisulfite (MSB) • 2.66 mg menadione nicotinamide bisulphite (MNB) • 2.22 mg menadione dimethyl pyrimidinol bisulphite (MPB)
-
-
-
Reduction of Pathogenic Bacteria in Animal Feed Using Organic Acids
Organic acids are increasingly utilized in feed safety management due to their proven efficacy in reducing Salmonella and other pathogenic bacteria in feed and feed mills. Through dietary acidification, they enhance environmental hygiene by safeguarding raw materials, compound feeds, and equipment from zoonotic agents. Their residual antimicrobial activity further mitigates recontamination risks.
Application in Feed and Water
Organic acids and their blends are applied via feed and water to sanitize and modulate gut microflora, thereby improving feed efficiency and reducing microbial load. Efficacy varies by substrate:
-
Pelleted and compound mash feeds: up to 2.5 log₁₀ reduction
-
Rapeseed meal: ~1 log₁₀ reduction
-
Soybean meal: <0.5 log₁₀ reduction
Formic and propionic acids, alone or with sodium formate, demonstrate the highest impact.
Targeting Salmonella spp.
The EFSA Panel on Biological Hazards identifies Salmonella spp. as a key microbial hazard in protein-rich and pelleted feeds. Acid blends—particularly formic:propionic (80:20)—show significant reductions:
-
Iba & Berchieri (1995): >1000-fold reduction in S. Typhimurium within 7 days
-
Field trials with 0.5% formic acid: Salmonella-positive breeder feed dropped from 4.1% to 1.1%
Strain-specific acid tolerance was observed, with S. Infantis being most resilient, followed by S. Putten, S. Senftenberg, and S. Typhimurium.
Broader Antimicrobial Spectrum
Organic acids also inhibit E. coli, Listeria monocytogenes, and Clostridium spp., though these are less commonly feedborne. Experimental findings include:
-
5 mmol/L propionic acid → transient bacteriostasis (30 min)
-
0.5–0.7 mol/L formic/propionic acid → ~90% E. coli mortality within 3 hours
-
Combined acids exhibit synergistic effects, notably in fish meal
-
-
<b data-start=”159″ data-end=”234″><strong data-start=”163″ data-end=”234″>Reduction of Pathogenic Bacteria in Animal Feed Using Organic Acids
Organic acids have gained increasing attention in feed safety management due to their ability to reduce <strong data-start=”340″ data-end=”354″>Salmonella and other pathogenic bacteria in feed and feed mills. Dietary acidification with organic acids contributes to environmental hygiene by protecting raw materials, compound feeds, and equipment from zoonotic agents. Their <strong data-start=”574″ data-end=”607″>residual antimicrobial effect also prevents recontamination.
<b data-start=”640″ data-end=”676″><strong data-start=”645″ data-end=”676″>Proposed Use in Animal Feed
Organic acids and their blends are used both in feed and water to sanitize and modulate gut microflora, improving feed efficiency and reducing bacterial contamination. The efficacy of acid treatments depends on the feed type. Studies show that <strong data-start=”921″ data-end=”950″>formic and propionic acid (alone or combined with sodium formate) achieve the highest reduction in <strong data-start=”1024″ data-end=”1078″>pelleted and compound mash feeds (up to 2.5 log₁₀), moderate effects in <strong data-start=”1100″ data-end=”1127″>rapeseed meal (1 log₁₀), and limited effects in <strong data-start=”1152″ data-end=”1181″>soybean meal (<0.5 log₁₀).
<b data-start=”1184″ data-end=”1221″><strong data-start=”1189″ data-end=”1221″>Combating Salmonella in Feed
The <strong data-start=”1226″ data-end=”1262″>EFSA Panel on Biological Hazards recognizes <em data-start=”1274″ data-end=”1291″>Salmonella spp. as a major microbial hazard in animal feeds, especially in protein-rich ingredients and pelleted feeds prone to cross-contamination. Studies demonstrate that formic acid and blends with propionic acid (typically 80:20 ratio) significantly reduce <em data-start=”1538″ data-end=”1550″>Salmonella counts. For example, <em data-start=”1572″ data-end=”1596″>Iba & Berchieri (1995) reported a <strong data-start=”1608″ data-end=”1632″>>1000-fold reduction in <em data-start=”1636″ data-end=”1652″>S. Typhimurium viability within seven days using formic–propionic acid blends. Large-scale trials using <strong data-start=”1742″ data-end=”1762″>0.5% formic acid reduced <em data-start=”1771″ data-end=”1783″>Salmonella-positive breeder feed samples from <strong data-start=”1819″ data-end=”1835″>4.1% to 1.1%.<br data-start=”1836″ data-end=”1839″> Among strains, <em data-start=”1854″ data-end=”1867″>S. Infantis showed the highest acid tolerance, followed by <em data-start=”1915″ data-end=”1926″ data-is-only-node=””>S. Putten, <em data-start=”1928″ data-end=”1944″>S. Senftenberg, and <em data-start=”1950″ data-end=”1966″>S. Typhimurium.
<b data-start=”1969″ data-end=”2003″><strong data-start=”1974″ data-end=”2003″>Effects on Other Bacteria
Organic acids are also active against <strong data-start=”2042″ data-end=”2053″>E. coli, <strong data-start=”2055″ data-end=”2081″>Listeria monocytogenes, and <strong data-start=”2087″ data-end=”2107″>Clostridium spp., though feed is a less common source of these pathogens. Experimental data show concentration-dependent inhibition of <em data-start=”2226″ data-end=”2235″>E. coli:
<ul data-start=”2239″ data-end=”2509″>
<strong data-start=”2241″ data-end=”2268″>5 mmol/L propionic acid → temporary bacteriostasis (30 min).
<strong data-start=”2310″ data-end=”2352″>0.5–0.7 mol/L formic or propionic acid → 90% bacterial death within 3 hours.
Combined use of formic and propionic acids exhibits <strong data-start=”2447″ data-end=”2483″>synergistic antimicrobial action, especially in fish meal.
-
Yes, by DEB